

Nanopore stoichiometry is a measurement approach for obtaining molecular information, with applications covering multiple significant fields such as molecular detection, molecular dynamics analysis, and precise measurement of biophysical processes and biochemical reactions. However, as a novel detection technology, nanopore is often constrained by factors such as the material nature of the pore and the electrolyte system.
In light of the crucial role that electrolytes play in regulating the nanopore electrical performance, Dr. Liang Wang et al., have delved into the impact of the Hofmeister effect on the stability and electrical detection performance of nanopores, revealing the physical mechanism of electrical parameters in regulating nanopore stoichiometry (ACS Materials Letters. 2025, 7, 7, 2476—2481. Featured on Cover). Recently, in order to further address the limitations of nanopore stoichiometry, Dr. Liang Wang et al. focused on the development of new biomaterials and constructed a novel type of anthrax nanopore device (Figure 1). Based on the similar mechanism of self-assemble anthrax protective antigen channel under acidic environment, we established an asymmetric pH gradient to allow the formation of functional anthrax nanopores by leveraging the proton-driven properties of the channel. The optimized anthrax nanopore demonstrated outstanding molecular detection capabilities, capable of distinguishing homologous proteins expressed by the key signaling pathway MAPKK gene family of eukaryotes. This research provides an effective solution to the limitations of signal attenuation and low-resolution in nanopore measurement. For more details, please see “Single-Molecule Sensing with an Anthrax Nanopore Enabled by pH-Asymmetric Ionic Liquids", Journal of Nanobiotechnology. https://doi.org/10.1186/s12951-025-03817-w
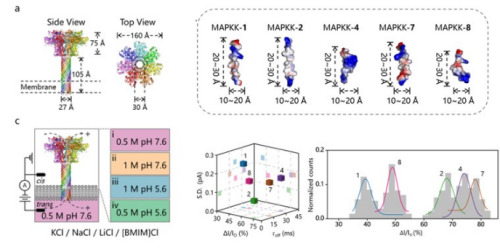
Figure 1. Single-Molecule Sensing with an Anthrax Nanopore Enabled by pH-Asymmetric Ionic Liquids
The binding of small molecules to DNA may represent a mutagenic process capable of inducing genomic structural alterations and functional impairment. Melamine (MA), a toxic small molecule, exhibits a hydrogen-bonding interface structurally analogous to adenine, enabling to form non-canonical thymine-melamine (T-MA) base pairs like Watson-Crick pairing(Nano Letters 2025, 25, 4, 1706–1714.). This property allows MA to program DNA nanostructure formation. Given MA’s documented biological consequences such as kidney disease, reproductive toxicity, and central nervous system dysfunction, sensitive detection of MA-DNA interactions has become critically important. However, such subtle structural changes remain challenging to be identified because of the paucity of effective detection approaches in high-resolution manner. To overcome this limitation, the scientists employed nanopore measurement to identify T-MA hydrogen bonding base pairing in DNA (Figure 2). Results demonstrated that nanopore enabled unambiguous identification of T-MA hydrogen bonding via mechanically unzipping thymine-melamine-thymine (T-MA-T) triplets in DNA structures. The approach achieves single-base-pair resolution, as evidenced by nucleotide substitutions flanking the abasic site in complex DNA structures. In addition, nanopore-based kinetic analysis revealed an enhanced intramolecular stability in MA-binding DNA compared to those consisting complete canonical DNA pairs. This research establishes a powerful platform for high-resolution interrogation of DNA-small molecule interactions and quantitative biophysical characterization of mutagenic modifications at the nanoscale. For more details, please see “Single-Molecule Nanopore Detection of Non-Canonical Thymine-Melamine Hydrogen Bonding Base Pair in DNA Abasic Site" https://doi.org/10.1002/smtd.202501445.
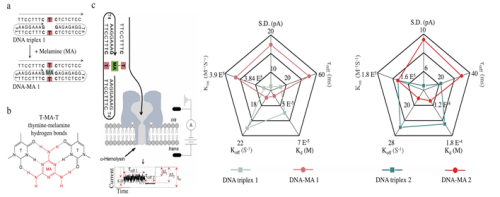
Figure 2. Nanopore identifies thymine-melamine-thymine (T-MA-T) hydrogen bonding pair within DNA at single abasic site level.
Dyshomeostasis of essential physiological metals and environmental exposure to heavy metals significantly influence both the functional and pathological alterations of Tau protein due to the structural changes. However, conventional methods for measuring metal ion-protein interactions are hard to achieve real-time monitoring of the protein aggregation at low-concentrations. The scientists track the aggregation processes and pathological conformational changes induced by nine essential physiological or environmental metals by analyzing differences in standard deviation of blockage amplitudes and duration times of current signals of nanopore measurement. This study provides novel approach for investigating Tau protein aggregation at nanoscale. The paper “Nanopore Measurement on Metals Induced Tau Protein Aggregation at Nanoscale”was granted Best Conference Paper Award in IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale 2025 (IEEE 3M-NANO 2025, Figure 3).

Figure 3. Best Conference Paper Award of IEEE 3M-NANO 2025.
Those works were supported by the National Key Research and Development Program of China (2022YFB3205600), Chongqing Talents: Exceptional Young Talents Project (cstc2021ycjh-bgzxm0016), Natural Science Foundation of Chongqing (CSTB2023NSCQ-MSX0071), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2022388), and National Institutes of Health (R01GM147247) and National Science Foundation (2345813).