


In the ever-evolving landscape of medical research, messenger RNA (mRNA) therapeutics have emerged as a promising frontier, holding great potential for applications in immunotherapy, protein replacement therapy, genome editing, and cellular reprogramming. However, the clinical implementation of mRNA therapies has been hindered by significant challenges, primarily centered around the efficient and safe delivery of mRNA. A recent groundbreaking study published in Advanced Materials by a collaborative team, including Associate Researcher Xiaobei Huang from the Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences, and Professor Dezhong Zhou from Xi'an Jiaotong University, presents a novel solution with the development of "four-in-one" highly branched poly(β-amino ester) nanocarriers (O-LhPAEs), revolutionizing mRNA delivery and offering new hope for the nebulization treatment of silicosis.
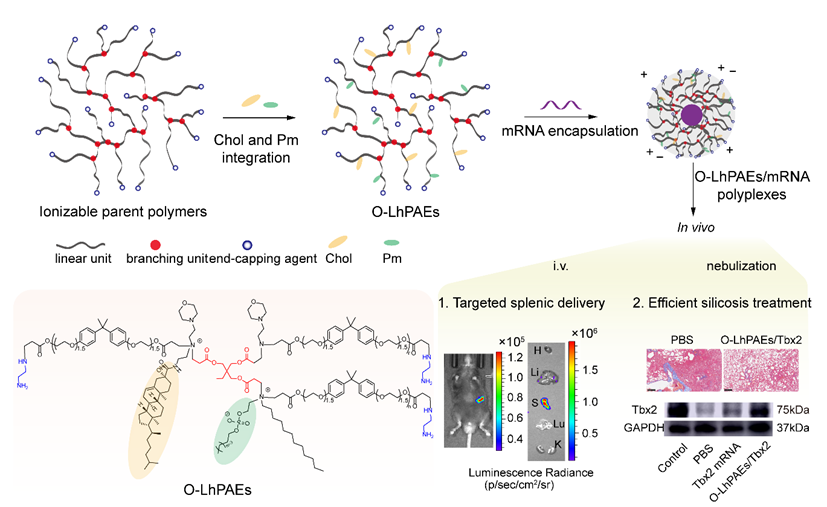
Figure 1. Development of “four-in-one” lipid-like O-LhPAEs for highly efficient spleen-selective mRNA delivery and nebulization treatment of silicosis in vivo.
mRNA therapies, despite their immense promise, face hurdles such as poor mRNA stability, low translational efficiency, and high immunogenicity in the human body. Conventional mRNA delivery systems, like lipid nanoparticles (LNPs), although showing some progress, suffer from issues such as complex fabrication processes and uneven distribution within the body. Against this backdrop, the research team embarked on an innovative approach, integrating the key components of LNPs into highly branched poly(β-amino ester)s. Through meticulous control over the branching structure and chemical composition, they successfully synthesized 60 distinct O-LhPAEs, which incorporate tertiary/quaternary amines, cholesterol moieties, zwitterionic species, and hydrophobic alkyl tails.
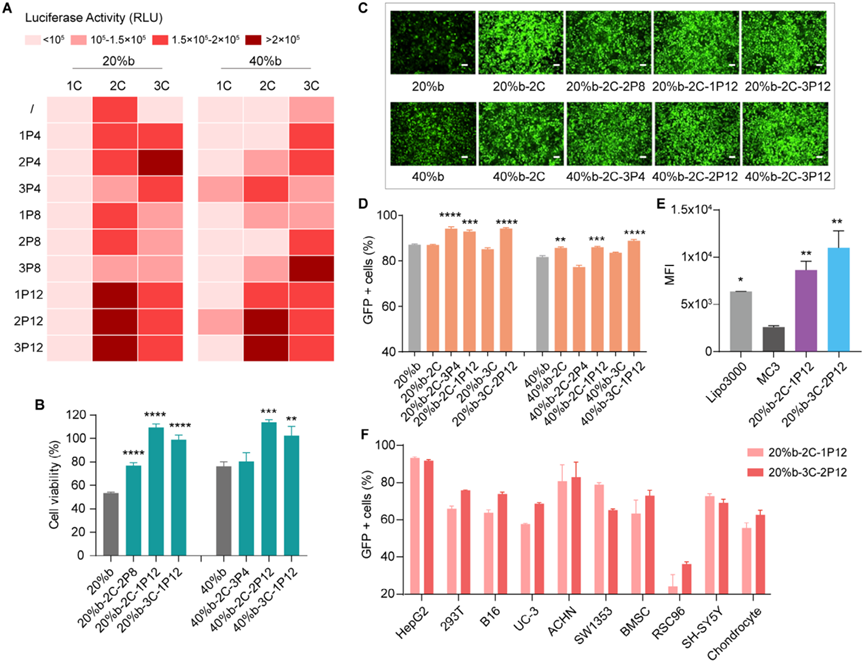
Figure 2. O-LhPAEs library exhibits superior mRNA transfection in vitro.
The new nanocarriers demonstrated exceptional performance in in vitro cell experiments. The optimal O-LhPAE, 20%b-3C-2P12, achieved an astonishingly high mRNA transfection efficiency of up to 93.1% across 11 different cell types, encompassing epithelial cells, fibroblasts, cancer cells, stem cells, neurological cells, and astrocytes. This broad adaptability across diverse cell types underscores the remarkable potential of O-LhPAEs to efficiently deliver mRNA into various cells, marking a significant advancement in the field.
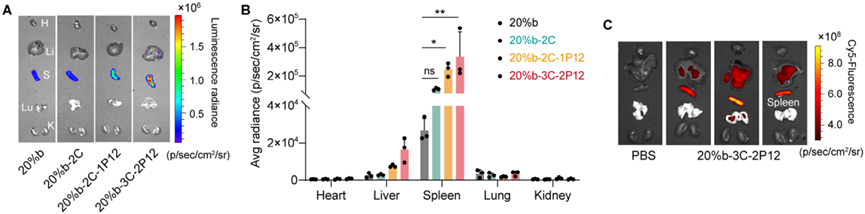
Figure 3. Biodistribution of O-LhPAEs/mRNA polyplexes.
In in vivo distribution studies, O-LhPAEs/mRNA polyplexes exhibited a unique characteristic of predominantly accumulating in the spleen rather than the liver. This distinctive distribution pattern is of great significance as it can potentially reduce off-target effects and facilitate the treatment of non-hepatic diseases. More intriguingly, through nebulization, 20%b-3C-2P12 enabled high expression of Tbx2 mRNA in the lungs of silicosis mice, effectively restoring their lung functions. This finding not only provides a novel strategy for mRNA delivery but also offers new therapeutic possibilities for lung diseases such as silicosis.
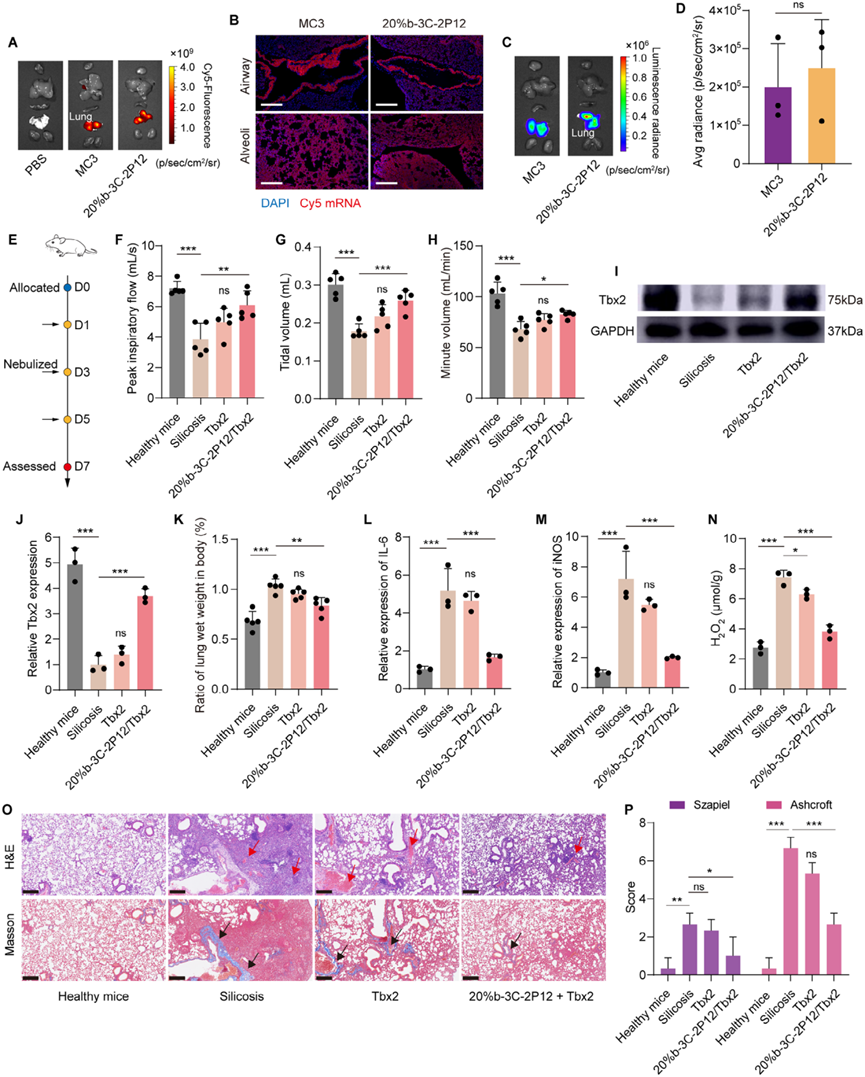
Figure 4. 20%b-3C-2P12 can effectively deliver Tbx2 mRNA for the treatment of silicosis through nebulization.
Safety and stability are also key strengths of O-LhPAEs. In cell experiments, the majority of O-LhPAEs maintained high cell viability after transfection, outperforming traditional mRNA delivery systems significantly. In in vivo experiments, mice showed no obvious toxic reactions after multiple nebulization administrations, further confirming the safety of O-LhPAEs.
This study not only opens up new avenues for the design and development of mRNA delivery systems but also provides strong support for the clinical application of mRNA therapies. By further optimizing the chemical composition and structure of O-LhPAEs, it is promising to develop more efficient and safer mRNA delivery systems, which may bring new breakthroughs in the treatment of a variety of diseases. In the future, these novel nanocarriers are expected to play a crucial role in the treatment of more diseases, paving the way for personalized and precise medicine.
The research was supported by several projects, including the National Natural Science Foundation of China and the Natural Science Foundation of Chongqing. This collaborative effort has undoubtedly set a new milestone in the field of mRNA delivery and holds great promise for the future of medical science.